We celebrate 15 years of ISO 13485 in 2025.


ISO 13485 Certified 15+ Years in a Row | World's First & Largest Custom SUT Library
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
Discover our North American designed and manufactured products.
Experience our Class 7 Clean Room—7 Rooms across 4 locations.
We have Class 7 & 8 molding capabilities.
Explore our cGMP Control for single-use manufacturing.
We celebrate 15 years of ISO 13485.
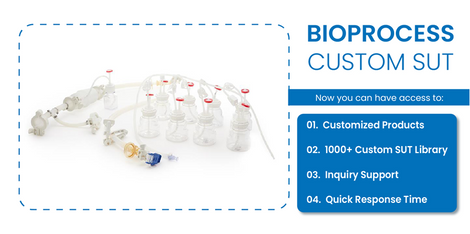
Discover EZBio® Single Use Media Bottles from Foxx Life Sciences—leak-proof, cost-effective, and versatile for sampling, media, and biologicals. Available in various sizes and caps to meet all application needs.
Learn MoreEZBio® 5L Erlenmeyer flask is E-Beam sterilized and ready-to-use, offering scalability, secure transport, and compatibility with incubator clamps for efficient microbial culture, media prep, storage, and sterile applications.
See All ProductEZBio® Carboys provide durable, leak-proof fluid storage and aseptic transfer for pharma, biotech, and lab applications, ensuring contamination control, efficiency, and scalability across various media preparation and fluid handling processes. .
See All ProductEZBio®pure Feed Systems provide sterile, single-use bottle-top filtration, ensuring contamination-free processing for cell culture, research, and pharma applications, while eliminating sterilization steps to save lab time and enhance workflow efficiency.
See All ProductEZBio® Ultra Bags offer high-performance, single-use fluid storage and transfer for critical bioprocessing applications. Made from medical-grade polyethylene, they ensure optimized flow, reliability, and customization for seamless, contamination-free operations.
See All ProductEZBio® Centrifuge Tube Assembly is a closed-system solution designed for biopharma sampling and transfer. Featuring TPE tubing and a vent filter, it ensures secure fluid handling with rapid MTO single-use delivery options.
See All ProductFoxx Life Sciences Selective BioProcess Tubing offers Fluoropolymer, Silicone, and TPE options, ensuring high performance for diverse applications. Designed for purity and compliance, each tubing type meets industry standards for safety and reliability.
Learn More

Thanks for subscribing!
This email has been registered!
| Product | SKU | Description | Collection | Availability | Product Type | Other Details |
|---|